Heisenberg's Uncertainty About People
Model of a hydrogen-like atom showing the probability distribution (S3) of the orbiting electron in blue. Source - Wikimedia, Geek3
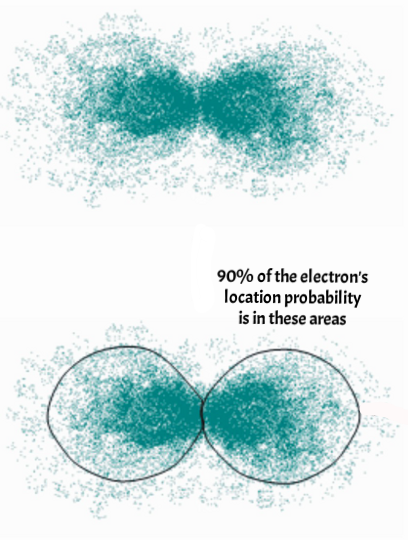
Model of a hydrogen-like atom showing the probability distribution (P2) of the electron in node form either side of the atoms' proton. Source - Wikimedia, CK-12 Foundation
Just to let you know up front, I'm talking about uncertainty at atomic and sub atomic level so that I can use it as a metaphor or analogy (can never remember which) for a particular characteristic of human interaction. As far as I am aware Werner Heisenberg was not especially uncertain about people, although he might have not bothered to tell me.
Among others Werner Heisenberg discovered some amazing things about how sub atomic particles behave and the consequences of trying to observe them. Electrons can sometimes behave as if they are a particle, and sometimes they can behave like a wave. Its called the wave/particle duality. As a particle it has a specific location, but when its a wave its on the move and doesn't really have a location, its more like a cloud of probability of where the particle could be, shaped like a wave. It behaves like a wave and so, unsurprisingly, it is a wave, which is why particles like electrons and photons can have a wavelength and a frequency. When a photon travels, say from a distant galaxy, it travels as a wave. When it encounters an immoveable object, like the light receptor in your eye, it collapses to a particle.
The usual way of showing an electron in orbit around a nucleus, like the orbit of the moon around the Earth is completely wrong. An electron's orbit is really a region of probability, a region where an electron could be if it decided to be a particle, as the two images left show.
For a hydrogen-like atom with one proton at the centre and an electron in orbit around it, different energy states create different arrangements of the electron's probability cloud. The first image shows the cloud as a ring, closely following the traditional location for an orbital particle but without a specific location (blue in the image). This is called the S3 orbital. The second image shows a P2 orbital, which one source described as like two balloons tied to together at the knots by the proton.
Unlike electrons traveling in wave mode from one place to another, the ones orbiting protons to make an atom are essentially standing waves, they're not going anywhere. We can't think of them as being particles, or that somewhere in those probability clouds there's an actual particle.
Unless.....
This is quantum physics, which is seriously weird, and this is one of the weirdest. If we start to look for an electron or a photon, the wave collapses down to a particle almost as if the electron is trying to accommodate the observer. It seems that the presence of an observer changes the dynamic of the environment and causes the electron or photon to change.
Although this weirdness has been known for over a century, its still not properly understood, prompting Richard Feynman to remark that if you think you understand quantum physics you don't understand quantum physics.
One issue connected to all of the above is Heisenberg's Uncertainty Principle. Heisenberg says that if we discover the location of a particle we can't know its velocity and vice versa. This is mainly because location is the particle's domain, and velocity (speed and direction) is the domain of the wave, and we can't observe sub atomic particles in both domains at the same time.
All this uncertainty has led people to conclude that making predictions is effectively impossible because we can't even predict what a photon or an electron is going to do next. This is not really true, and I will try to illustrate this by introducing another level of uncertainty, the movement of molecules in a gas.
The mindless and unpredictable movement of gas molecules
If the volume of a body of gas is halved, the pressure doubles. Source - Wikimedia, cnx.org, OpenStax College
Gas molecules are constantly moving. If they move more vigorously they register a higher temperature. If they move more sluggishly they register a lower temperature. If they stop moving altogether they register a temperature of 273.15 degrees centigrade below zero. This is called absolute zero. Nothing can be colder than absolute zero because the movement of atoms and molecules IS temperature. It is not a secondary characteristic. The sensation of heat or cold is directly caused by moving atoms and molecules.
If the temperature in a container of gas is increased, the pressure also increases. In this illustration extra weights are needed to keep the volume steady. Source - Wikimedia, Egmason
The thing about this movement though is that its random and unpredictable for individual atoms and molecules, but when a large collection of them get together in a container their collective behaviour can be predicted.
In a sealed container filled with a gas there is a direct relationship between temperature, pressure and volume, despite the unpredictability of individual gas molecules we have accurate formulas for the way these three things change relative to each other. The laws are variously called Boyle's Law, Gay Lussac's Law, the Pressure Temperature Law and the Pressure Volume Law.
If the volume of a gas is halved the pressure doubles. The two measures are inversely proportional.
If the temperature of a fixed volume of gas is increased the pressure of the gas also rises. The two measures are directly proportional.
So to summarise
Sub atomic particles can also be waves.
Particles have location, waves have velocity.
We can't know both at once.
An observer of sub atomic particles can cause a change of state (particle or wave) depending on the observer's behaviour.
In other words the presence of an observer changes the dynamic.
Despite being unpredictable, sub atomic particles, atoms, and molecules can become predictable in large quantities by studying the external consequences of their collective unpredictability.
This is done by measuring, seeking patterns in their behaviour and creating formulae and laws that both describe them and render them predictable.
So what has this got to do with human interactions?
A person on their own does whatever a person on their own does. Then another person enters the first person's space. This is a new dynamic and both people change their behaviour to accommodate it. This means that neither person truly knows the other in their natural setting, only in the setting that includes them. You could describe the dynamic as a description of the space between the two people. It has its own unique character specific to those two people. If the two people were a couple sharing a home there will be three different dynamics. Each of the two on their own and when they are together. It is in this sense that we can't completely know even those who are closest to us. Then they invite another couple to dinner and everything changes. There is the collective four-person dynamic, four separate three-person dynamics, and six two-person dynamics. You can imagine how the relevant ones ebb and flow as people move around and regroup. Anyone who goes to the toilet or pops up the shop for some more wine becomes their natural selves for a while. The differences may not be all that great, but they will be there.
If only four people can create such interactive complexity then crowds should be and usually are a nightmare to fathom.
An example of this phenomenon, domestic violence, has been topical for a long time and many are saying it has reached epidemic proportions. One of the most common comments you hear from family and friends is "They seemed like the perfect couple." This is an impression that can go on for many years, especially if the abuse is hidden (no broken bones or bruising). A recent news item talked about one example in which even close family were unaware for 17 years. We don't think about how our close friends behave when we're not around and in the vast majority of cases we don't need to. But domestic violence professionals will tell you that there are signs if you are trained to spot them. The medical website WebMD lists these external signs:
Excuses for injuries
Personality changes, like low self-esteem in someone who was always confident
Constantly checking in with their partner
Never having money on hand
Overly worried about pleasing their partner
Skipping out on work, school, or social outings for no clear reason
Wearing clothes that don’t fit the season, like long sleeves in summer to cover bruises.
There is also the effect of witnessing domestic abuse in children. For example changes in behaviour at school.
Another medical website, www.emedicinehealth.com also lists signs from the abuser. Controlling behaviour, answering all the questions, not leaving the victim alone with others etc.
What this demonstrates is that it is possible to identify hidden relationship issues by looking for clues and patterns, just as our understanding of the laws that govern the behaviour of gasses were derived from observing external changes. Another example from science is the way we understand the nature of black holes. We can't observe them so instead we observe their external effects on the surrounding space. This technique is so good that we can determine, not just location but size, spin, gravitational pull, structure etc.
We are also slowly getting better at understanding the dynamics of crowd behaviour, almost as if we are treating it in a similar way to the behaviour of large collections of molecules. There is a branch of psychology devoted to the understanding and prediction of crowds. The Wikipedia page on crowd psychology quotes the Corsini Encyclopedia of Psychology thus:
Crowds can be active (mobs) or passive (audiences). Active crowds can be further divided into aggressive, escapist, acquisitive, or expressive mobs. Aggressive mobs are often violent and outwardly focused. Examples are football riots and the L.A. riots of 1992. Escapist mobs are characterised by a large number of panicked people trying to get out of a dangerous situation. Acquisitive mobs occur when large numbers of people are fighting for limited resources. An expressive mob is any other large group of people gathering for an active purpose. Civil disobedience, rock concerts, and religious revivals all fall under this category.
While we cannot know how individuals and couples behave alone in their natural environment, we can know them by how they behave in our presence or in public. For most of the time this is as much as any of us need. It is only when the external signs suggest there are troubling things happening in private that we need to pay more attention.
Confession
I started by saying that I was using sub atomic uncertainty as a way of talking about human interaction. This is only partly true. For a long time I have wanted to tackle this aspect of quantum physics, just to see if I could succeed in understanding a complex subject well enough to avoid mistakes and make it easier to understand. I have no idea if I achieved that, so let me know what you think.