The Enduring Facilitator
You walk into a room occupied by two people. You force the first person to exchange clothes with you, then you force that person to exchange clothes with the second person. You then exchange clothes with the second person, and leave the room as the only person with his original clothes on. Although it is not strictly true that you haven’t been changed at all by the experience, in human terms you have just been a catalyst.
A catalyst facilitates change and emerges unscathed. It does this by providing pathways that either force, encourage or speed up change. The important consequence of this scenario is that the catalyst can then repeat the action, in the case of the example above, by barging into another room with another unsuspecting couple. She could do this several times a day as a hobby.
Consilience
Edward O Wilson is one of the most famous and respected scientists in the world. Whilst his discipline is biology he has written frequently across the scientific spectrum. In his book ‘Consilience: The Unity of Knowledge’ he resurrected the word consilience from obscurity, and used it to describe the way the different disciplines of science collaborate to pursue particular areas of knowledge. In this collaborative work it is often possible to find threads that bind the disciplines more closely with common underlying principles. Working across disciplines is something that happens often in the natural or ‘hard’ sciences like physics, chemistry biology, and geology, and I have written about this here in the article “Knowledge Fans.”
Wilson’s book concentrates most on the failure of the social sciences like anthropology, sociology, history, geography and economics to work together in the same way, and in particular the difficulty of breaking down the barrier between the ‘hard’ natural and ‘soft’ social sciences so that they can profitably collaborate in the study of some aspects of the physical world. The book is a revelation and I can highly recommend it.
Catalysts
One example of how our understanding can track across disciplines and cross the barrier between natural and social sciences is when emerging descriptions can not only use the same words or phrases, but tantalisingly suggest an underlying law or principle that connects them all. Critical mass would be an example of this. In this case though we are looking at catalysts which are found in chemistry, molecular biology, species interactions and human society.
How the catalytic converter works. Source - Science Learning Hub – Pokapū Akoranga Pūtaiao, University of Waikato, www.sciencelearn.org.nz
Chemical Catalysts: The Catalytic Converter
Car exhausts produce, among other things, carbon monoxide, which is very bad indeed. It is highly toxic as well as being a minor and indirect contributor to the greenhouse effect. A catalytic converter combines the carbon monoxide with oxygen to produce carbon dioxide, which although a greenhouse gas is not locally lethal (so subdued applause please). Unfortunately oxygen molecules (O2) and carbon monoxide (CO) won’t oblige by combining if left to their own devices.
So platinum comes to the rescue. The two molecules bind lightly to the platinum. This is sufficient for the two atoms of the oxygen molecule to separate and combine with two carbon monoxide molecules to form carbon dioxide. This is an example of a catalyst (platinum) that doesn’t get involved in the chemical changes directly, but provides a base that weakens molecular bonds enough to facilitate the change.
Car exhausts also produce harmful hydrocarbons and nitrous oxides. the elements palladium and rhodium act as catalysts for these, converting them to water carbon dioxide, nitrogen and oxygen.
This image shows the resulting chlorine monoxide atom before it sheds the oxygen and becomes a catalyst able to repeat the process. Source Flickr - Siyavula Education
Chlorine and ozone depletion
Not all catalytic chemical reactions are useful or benign. The chlorine in CFCs Is a pretty scary example of this.
CFCs or chlorofluorocarbons are non toxic and nonflammable and have been used for aerosol sprays, solvents and refrigerants until recently. They were phased out because of the damage they were doing to the ozone layer. Ozone is a molecule with three oxygen atoms instead of the usual two. The ozone layer is vital because it protects life on our planet from harmful ultraviolet light. Once CFCs get into the ozone layer, about 25 kilometres above the surface, ultraviolet light splits chlorine off, which then pinches one of ozone’s oxygen atoms. This converts the ozone to bog standard oxygen and chlorine to chlorine monoxide (see image left).
This wouldn’t be too bad, but we haven’t got to the scary bit yet. The chlorine sheds its newly acquired oxygen molecule and returns to it original state. This makes it a catalyst available to do the same thing all over again. On average each chlorine molecule converts around 100,000 ozone molecules to oxygen. This is why climate scientists say that CFCs are dangerous because they take such a long time to dissipate. A chemical catalyst can only be stopped from repeating its process through some form of attrition, like drifting off into space, or being zapped by a cosmic ray.
The “lock and key” mechanism. The substrate on the left is split after binding with the enzyme to two new molecules. For example sucrose to fructose and glucose. Source -Wikimedia, TimVickers
Biological Catalysts: The Enzymes
Catalase: the enzyme that breaks down hydrogen peroxide to water and oxygen. Source - Wikimedia, Vossman
There are around 75,000 enzymes in life on earth and without them life would not be possible. Enzymes facilitate most of the reactions in our bodies that sustain life, once again without being changed themselves.
The first image left shows the principle at work. The enzyme is structured in such a way that it has an exact shape that only one particular molecule in the body can attach to. This is why there are so many enzymes. Each one has a single job and there are lots of such jobs in the body, each one requiring a different shape. The illustration shows the enzyme with a cavity, but it can also just as easily be a projection depending on the job. The illustration also shows an enzyme breaking a large molecule which the body can’t use (sucrose) into two smaller molecules (fructose and glucose) which it can use. But enzymes can also combine smaller molecules into larger ones. It’s horses for courses.
The second illustration is a ribbon diagram of the Catalase enzyme (all enzyme names end in -ase). Catalase is found in all life on earth that is exposed to oxygen. This is one of the thousands of processes that demonstrate the common ancestry of all life on Earth because they are found in all species. The first thing to notice is how complex it is. Enzymes are one class of proteins and all proteins are constructed from strings of amino acids. There are around 2000 amino acids in the Catalase chain.
The interesting thing is how and why the molecule forms itself the way it does. It looks like a roughly crumpled up length of string, but appearances are deceptive. Only a very small part of the molecule is active in the catalytic process, basically the amino acids surrounding the capture site. But the capture site has to be the exact shape required to carry out its function and the rest of the molecule is responsible for arranging itself to guarantee that shape.
Hydrogen peroxide is water, H2O, with an extra oxygen atom, H2O2. The body manufactures H2O2 to control the dangerous substance, superoxide. Unfortunately too much H2O2 is also bad because its products can attack proteins and DNA. The role of the catalase enzyme is to control the amount of H2O2 by breaking it down into water and oxygen. Catalase has one of the highest turnover rates of all enzymes, able to convert millions of hydrogen peroxide molecules per second.
Just as a point of interest, enzymes are destroyed by cooking. Whether this is a good or a bad thing is controversial. One school of thought says that the enzymes in food are for the use of the food item, not for us, and that our body has its own enzymes for that purpose. The opposite view is that only humans cook their food and in any case didn’t always do so. The enzymes in raw food predigest while in the stomach, putting less strain on the body as a result. I’m taking no sides at this point and you would never get me to eat raw meat anyway.
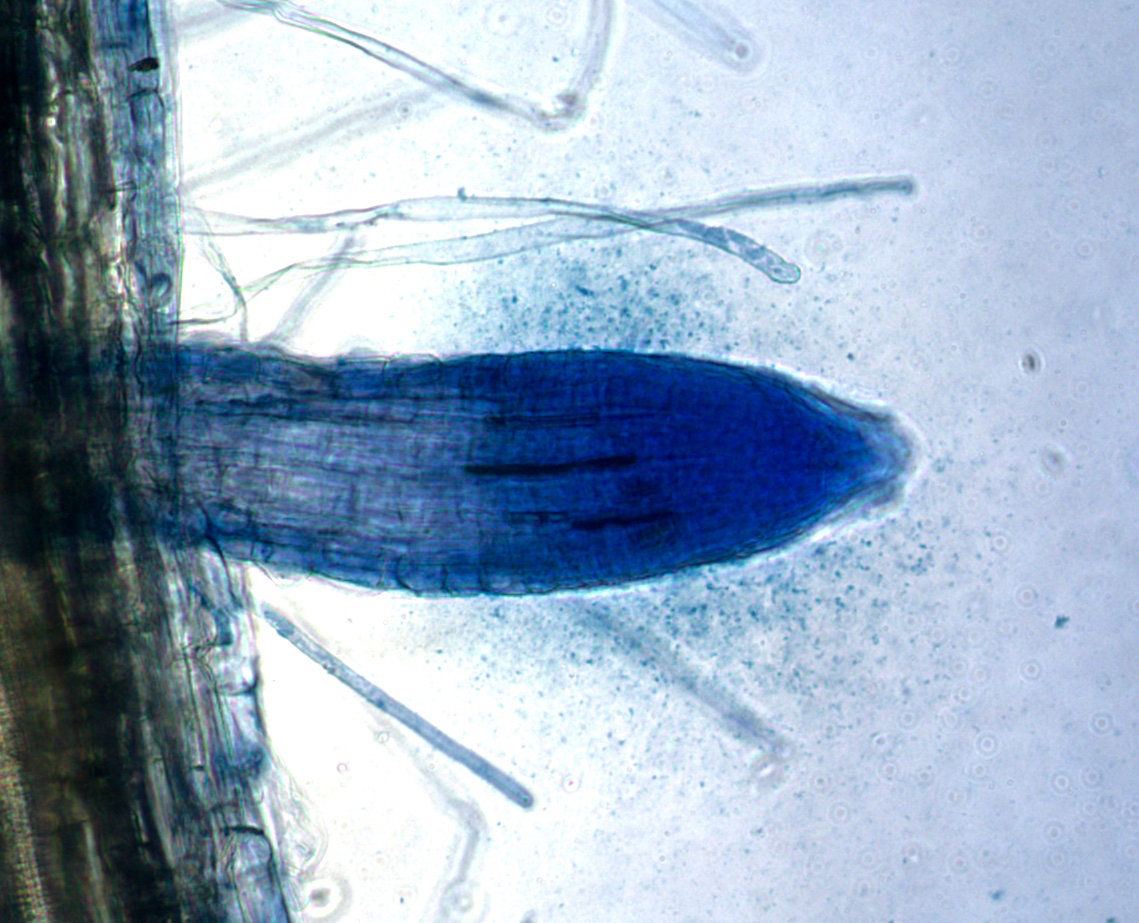
Bacteria facilitate nutrient absorption by plant roots. Source - phys.org, Professor James F. White Jr./Rutgers University-New Brunswick
Species Catalysts: Rhizophagy
A root of the common reed showing bacteria in a cloud around the root tip. Source - phys.org, Professor James F. White Jr./Rutgers University-New Brunswick
The rhizosphere is the soil immediately surrounding roots of plants. The soil contains bacteria which are catalysts for the absorption of nutrients by the roots.
The diagram left shows the sequence of events:
Bottom centre - The bacteria have absorbed food from the soil. The food is supplied mainly by the plant root excretions which are then transformed by the bacteria. This ties the bacteria to the plant.
Centre right - The bacteria enter the root tip. The image top right (B) shows the bacteria in the root cells of the tip.
Centre - The root extracts the processed nutrients from the bacteria.
Left - Bacteria emerge by stimulating the formation of fine hairs further up from the tip and leaving from the tip of the hairs. They are then available to start the process all over again.
One of things I like about this is that the bacteria seal the exit before departing, effectively shutting the door behind them.
The discovery of this symbiotic relationship is relatively recent and work is already underway to find out if this process can be exploited to increase crop yield, battle weeds and drastically reduce the need for herbicides and fertilisers.
There are many such catalytic and symbiotic relationships like these that involve single celled life. I have not been successful yet in finding similarly catalytic relationships between multi-celled animals, although I am sure they exist. If anyone knows of any, let me know and I will include them here.
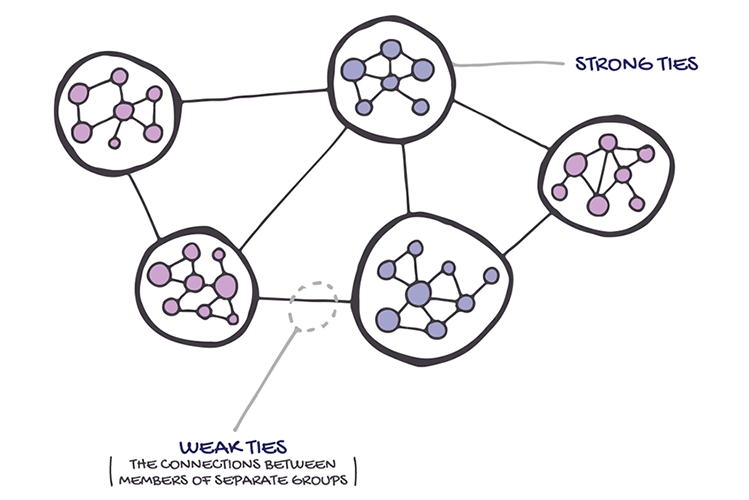
The strength of weak ties. Source - https://blog.headresourcing.com/networking-and-the-strength-of-weak-ties/
Human Catalysts: The Connectors
One of the most interesting things to come out of the study of social networks is that it consists mainly of clusters, groups of people that all know each other. Potentially these clusters could be isolated, like islands scattered in a vast ocean, unable to bridge the gaps between them. Why this doesn’t happen is even more interesting
In my essay “Knowledge Fans” I used something called ‘The Six Degrees of Separation’ to explain aspects of connectivity in knowledge. This shows that any two randomly chosen people from anywhere in the world can be connected through friends and acquaintances by an average of around 6 steps. But if people cluster (and they do) how is this short number of steps possible? The answer is that members of one cluster are also members of other clusters. In 1973 the American sociologist Mark Granovetter published a paper called ‘The Strength of Weak Ties’ Showing why the individuals that connected clusters to each other were so important to the general connectivity of social networks. I’ve included the video I used in the other essay because it explains this really well.
But a passive connector doesn’t really do very much. In fact the members of his or her different clusters may not even know about each other. But a proactive connector can justifiably be called a catalyst. If you are the kind of person who likes to bring friends that don’t know each other together you are changing the dynamic of the groups they belong to. You’re a catalyst. On occasions when I have organised social events, I have invited people from across the social groups I belong to and some long term friendships have arisen that might not have taken place. The fact that I love to do this has made me wonder if I might be a connector too.
This type of catalyst can be summed up by saying that they facilitate fruitful meetings between people who would not normally encounter each other or even link up if they did.
The Stirrer
Some people are just trouble makers. The person at the top of this article is an example of somebody who stirs things up then walks away, probably with a maniacal grin on their face and a hunger for more chaos.
Lots of people stir things up. We all have an impact on the fellow humans we come into contact with, but most times we are part of the changing dynamic so we don’t emerge unscathed. Being responsible for something doesn’t necessarily mean you are its catalyst.
A disruptive catalyst can be the facilitator of big changes that can be either good or bad. A group of people that have evolved into a moribund and repetitive complacency can be fired up to great things by a random bit of disruption. A bit like a cosmic ray mutating a piece of DNA. Some good stirrers do it strategically to spark creativity. On the bad side, I have personally witnessed the damaging effect a single disruptive person can have on an organisation. In the example I’m thinking of the culprit definitely left unchanged, no doubt to do the same thing in their next job.
The Mediator
Some mediators are professional catalysts, and it could be argued that getting paid to mediate means they are changed, but I think that’s a bit anal. Counsellors for example act as an unbiased catalyst providing the environment and stimulus for people to work out for themselves the issues they face, whether it is for a troubled individual or a couple with a relationship problem.
An example of unpaid proactive mediation is provided by yourdictionary.com, although it probably isn’t a true story:
Jennifer and Jason met in Mrs. Harrison's 1st grade class. They were friends all through grade school, high school, and college. Their friends and family members all thought that they should date, but they never did, and the people around them began to get impatient. Finally their friends Sarah and Matt took the matter into their own hands. Sarah asked Jennifer to go to a concert with her, and Matt asked Jason to go to the same concert with him. At the last minute Sarah and Matt cancelled, leaving Jennifer and Jason to attend the concert together. They began dating and later got married. Sarah and Matt were the catalysts in bringing about Jennifer and Jason's relationship.
Even if Jennifer and Jason didn’t start dating the catalysts still set the scene. They didn’t force the issue but created circumstances in which it could happen.
The Parental Catalyst
I referred to this next example in the very first essay on this site Nurturing the Thirst for Knowledge. Parents can be the catalysts for the the development of their children. The urge for parents to point their children towards their own preferences, profession, beliefs etc is naturally strong and unsurprising. This type of parenting is not catalytic. The parent who is a catalyst for their children’s development is the one that creates, populates and oversees an environment for the child to make it’s own personal journey towards adulthood. A journey without preconditions or preconceptions other than safety and universal values that are independent of ideology.
There is a deep joy in seeing your children and grandchildren explore the world around them only with a few but necessary boundaries. In my experience you can’t see the living room floor by midday. Many’s the time I have had to walk around the outside of a room to go to the toilet.
An Emergent Property
An emergent property is one that arises from complex systems where the individual parts do not have that property. An example would be an ant colony that has collective properties that the individual ants do not possess. I’m not sure if on this basis I can claim catalysis is an emergent property, but I’m going to do it anyway. tell me if you think I’m wrong.
Catalysis is an emergent property that doesn’t recognise the boundaries of scientific disciplines and can be found arising from a wide range of physical systems. Does this mean that there is an underlying process at work that is common to all scenarios? To be honest I’m not sure, but I’m going to suggest one possibility.
Everything evolves, not just living things. The difference is the mechanisms that cause the evolution are not biological. To use a daft example, a clay pot left entirely to its own devices for a million years in the open will gradually crumble to dust. The mechanism in this case is entropy and the passage of time. I will no doubt write an essay on entropy at some point.
It occurs to me that catalysis is the epitome of efficiency. To be able to carry out a function repeatedly with the same agent is massively more efficient than having to employ a new agent for each operation.
Enzymes It is certain that enzymes have evolved to their present competence and repetition is the evolved and emergent solution. Imagine how it would be if every break up of a hydrogen peroxide molecule required a brand new enzyme to be generated from scratch.
Rhizophagy The species interaction example has also evolved along with the well understood evolution of symbiotic relationships.
Chemical catalysts The periodic table reveals characteristics of the different elements that determine how they combine and split to form new molecules in chemical reactions. Whilst they have clearly not evolved to have these characteristics, we can say that if a particular reaction is possible it will take place if the elements are present and the environment favours it. I’m pushing my luck here to suggest that the evolving part of the equation is the favourable environment for a given chemical reaction.
People I’m not sure of my ground with the human examples either, except to suggest that society now evolves at least as much as our physical bodies and possibly more. Because catalysis in the social context is mostly beneficial, biological and social evolution should together evolve our behaviours to be more catalytic.
Well that was fun. I’ll probably do critical mass next for this theme.